Biosafety and Facilities Support
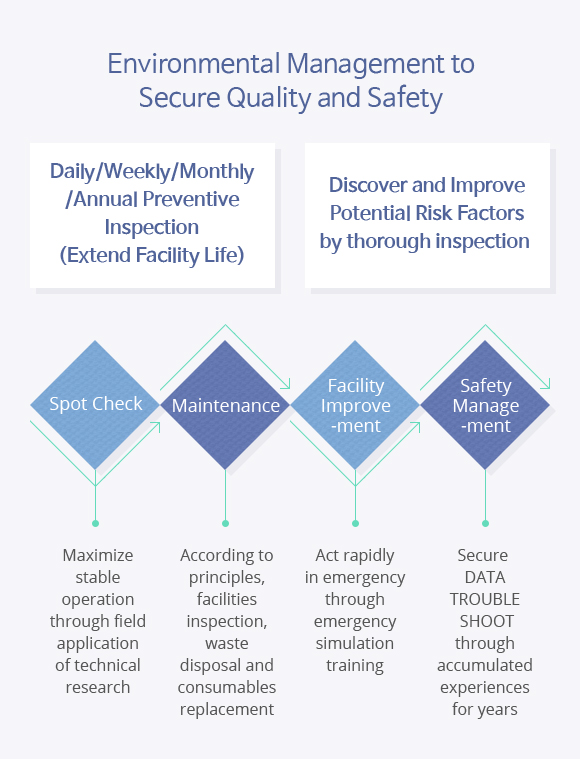
GCEM is providing customers with maintenance service through the newest technology including effective and efficient facilities operation and training for users to maintain the optimum condition of original facilities’ functions based on maintenance experiences of pharmaceutical facilities, research facilities, Negative Pressure Isolation Rooms, etc.
All tasks with being divided into groups of two proceed with establishment of plans, report of schedule, task performance, report of completion and storage of record according to the Safety Management Guidelines and the Standard Operating Procedures.
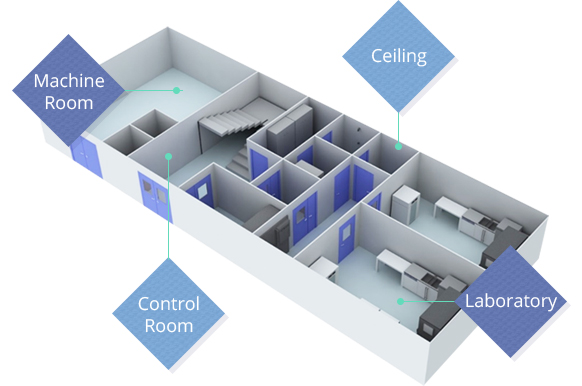
Machine Room
- AHU Management
- EF Management
- Chemical System Management
- UPS Management
- Control Panel (DDC)
- Consumables Replacement
- Disposal of Waste Water
Ceiling
- HEPA FILTER Management
- Validation and Calibration of Measuring Instruments
- Damper and Valve Check
- DUCT Confidential Management
- Inspection of Completion of Facilities
Control Room
- HMI Management
- Current Status
- Past History Management
- Alarm Management
- Data Management
- Access Control
- CCTV
Laboratory
- Temperature, Moisture, Differential Pressure Check
- Number of Ventilation and Sealing Check
- BSC Check
- Pass Box Check
- AUTOCLAVE Check
- Door Interlock Check
- Fumigation & Cleaning
- Emergency Reaction and Action

