
Conceptual design is the first task in design phase of GMP project, which is to establish “the Optimized Production System” through “optimized Layout” on the basis of User Requirements Specification and GMP (Good Manufacturing Practice) requirements.
Project Cost Influence Curve
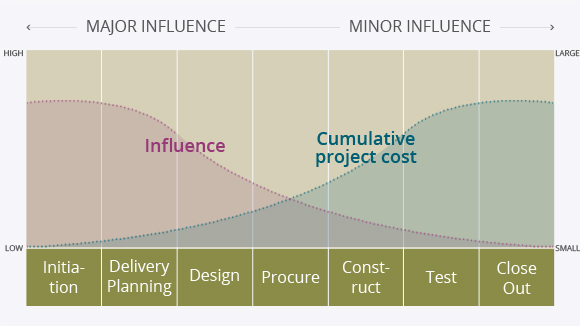 Sources : ISPE Good Practice Guide
Sources : ISPE Good Practice Guide (Project Management for the Pharmaceutical Industry)
In conceptual design phase, many risk factors such as regulation, business plan and cost plan should be considered to meet project deadline and minimize project cost. If you found errors later with regard to regulations/business plan/investment plan, It may cause extension of period and increase in cost.
GMP Coordinator
GCEM has state of the art technology derived from a lot of experience and co-work with global engineering companies.
GCEM’s QUALITY MANAGEMENT TEAM offers professional technical advice, customer based conceptual design and project coordination service depending on business needs.

Conceptual Design Execution
Procedure
In the conceptual design phase, we confirm the layout via adjustment and arrangement of required rooms in consideration of GMP flows and production capacity.
We also provide utility concept necessary to operate production area and production support area.
-
STEP
01
Kick off Meeting Planned project approach
- Select personnel to deliver documents and information
- Discuss mutual communication methods
- Discuss conceptual design schedule
- Share user requirements specification
-
STEP
02
Process Clarification
- Share production process information
- Organize the optimum production process flow
- Confirm requirements for production equipment
-
STEP
03
Areas Clarification
- Establish GMP flows (P/M/P/W FLOW)
- Arrange optimized process equipments
- Room Classification Plan
- Pressure differential Plan
- Gowning Plan
- Layout confirmation
-
STEP
04
Production supporting facilities
- Clean utility plan
- Architectural concept
- Mechanical concept
- Electrical concept
-
STEP
05
Strategy to Comply with GMP
- Strategy to ensure compliance with GMP
- Strategy to validation plan and schedule
-
STEP
06
Final Report of Conceptual Design
- Final report of conceptual design
- Submit Conceptual design package
Concept Design Deliverable List
GCEM is performing conceptual design service by developing execution strategy and document system of conceptual design through convergence of domestic and international GMP guidelines and engineering technology.
-
- Project Execution Plan
- Minute of meeting, Meeting agenda
- Project schedule
-
- Clean Utilities Concept report
- Clean Utilities schematic
-
- Electrical Concept Report
- Power Incoming Concept
- Single Line Diagram
-
- Process Concept Report
- Preliminary Process Description
- Process Block Flow Diagrams
- Preliminary Process Flow Diagrams
- Preliminary Process Equipment List
- Preliminary Process Equipment Layout
- Hygienic and Gowning Concept
- Validation & GMP Compliance Concept
-
- Architectural Concept report
- Site Plan
- Logistics Plan
- Floor Plan
- GMP Flow Diagram(P/M/P/W)
- Section Plan
- Preliminary Room Data Sheet
- Preliminary Bird's-eye view
-
- Mechanical concept report
- Air handling unit zoning plan
- Class zoning floor plan
- Room differential pressure plan
- HVAC system schematic
- Plant steam schematic
- Cooling system schematic
- Heating system schematic
- Potable Cold/Hot water system schematic
- Fire protection concept
- Waste water treatment concept
- Building automation system schematic
